Strong scientists, researchers and strategists, mostly with MD, PhD or both, more than 20 years of experience, and a comprehensive understanding of the interaction between science, innovative clinical trial design, and the commercial, regulatory and operational considerations that contribute to successful drug development across a wide range of organizations from startups through to mature biotechnology companies.
Our international team is made up of highly experienced drug developers

What Adnovate means to us
The word ‘Adnovate’ is formed from the two Latin roots ‘ad’ meaning towards, and the verb ‘innovare’ meaning to innovate, reform or change. Combined they represent the concept of advancement towards desired outcomes using innovative thinking and processes to maximize value creation for our clients.
Adnovate is a science-led clinical development consultancy servicing the life sciences sector. We have expertise in all phases of drug development, and across a wide range of platforms and therapy areas.
CLINICAL DEVELOPMENT EXPERTS
Strong scientists, researchers and strategists with MD, PhD or both and decades of drug development experience
GLOBAL
NETWORK
An extensive international team of experts from core clinical and translational development functions
INDUSTRY
LEADERS
Ex-leaders from Pharma and Biotechnology companies including the startup sector
The Executive Team
Our executive team brings together a wealth of global leadership experience and expertise in strategic and operational drug development, and corporate R&D structuring and functioning.

MBChB, MRCP, DPM
Previously a practicing physician trained in internal medicine and nephrology with >20 years of drug development experience from Pharma and Biotech organizations. Prior to founding Adnovate Clinical, Ajay was VP and Global Therapeutic Head General Medicine at Takeda and CMO/Interim CMO at several early-stage biotech companies providing leadership and scientific/technical expertise to deliver Medical and Clinical Development Strategies and oversight for operational execution to advance asset development. Experience spans across a wide range of therapeutic areas and all phases of drug development, including oncology, inflammatory and immunological based indications, and translational programs with cell and gene therapies, biologics and small molecules.

MSc
International biopharma leader driven by a deep commitment to improving patient outcomes. With more than 25 years of experience across drug development, commercialisation, and portfolio strategy in the pharmaceutical and biotechnology sectors, brings extensive clinical and commercial expertise. Has led organisations across the full therapeutic lifecycle—from translational research and clinical development through to commercial planning, portfolio management, market access, capital strategy, and investor relations to launch execution, LOE strategy, and complex crisis management.
Previous roles include Managing Director and CEO of HaemaLogiX and Race Oncology; U.S. Executive Director and Head of the Global Launch Leadership Team for leniolisib (Joenja) at Pharming Healthcare in rare disease; and Global and US Executive Marketing Lead in Neuroscience at Novartis, where the franchise was built on his lead asset fingolimod (Gilenya) delivering US$3.4 billion in annual revenue. Additional executive leadership roles have been held at Novartis, Zimmer, Celgene, and Biogen across orthopaedics, immunology, neuroscience, and rare disease.

MBChB MRCP
Highly experienced industry physician and executive with over 30 years experience in both global Pharma and biotech start-up organizations in UK and USA. Joined Pfizer from the UK NHS and held positions of increasing responsibility in GI/GU, anti-infective and sexual medicine therapeutic areas before becoming Head of Clinical Neuroscience at R&D HQ in Connecticut USA. Was Head of Clinical and Analytical Science for Takeda in Europe with responsibility for all TAs plus Clinical Pharmacology, Biometrics and Medical Writing and has also been CMO in multiple biotech organizations, most recently CMO and EVP Development in Cambridge UK. Has been a major contributor in multiple IND/CTA,NDA and MAA submissions and approvals across multiple TAs including GI/GU, anti-bacterial, antifungal, cardiorenal and neuroscience.

MBA, MSc
Life sciences executive with over 25 years’ experience in the pharma/biotech industry. Provides strategic advice and solutions to a diverse range of clients, from start-ups to Fortune 500 companies, leveraging a Harvard Business School MBA, a MSc in Health Economics, Epidemiology and Clinical Evaluation and a BA in Philosophy, Politics and Economics from Oxford University to deliver high-quality and impactful results. Experience across a range of therapeutic areas including oncology, neurology, and rare diseases, and across a range of ATMP modalities including gene and cell therapies. Core competencies include strategy, management consulting, strategic consulting, leadership, communication, problem-solving, and analytical thinking. Passionate about helping organizations achieve their goals to create a positive impact on patients, their families and healthcare systems.
The Operations Team
Our Operations team ensures Adnovate runs seamlessly, enabling our scientific experts to focus on what they do best. We manage the essential infrastructure that supports client engagement. By optimizing internal processes and maintaining operational excellence, we create the foundation for our team to deliver flexible, high-quality consulting services that adapt to our clients' evolving needs.

BA, ACA
Forward-thinking, strategically-minded Chartered Accountant with over thirty years of experience in senior financial leadership positions across a broad range of businesses including life sciences. Extensive experience in Financial Reporting & Analysis, Strategic Planning, Mergers & Acquisitions, Operations Management and Business Relationships. Established professional record of developing and implementing business plans, exercising financial rigor and due diligence to business proposals and strategy, leading major commercial and financial projects including cost rationalization programs, mergers, acquisitions and disposals. Proven ability to operate as business partner and provide commercial and financial oversight at board level. Previous positions include fifteen years as Finance Director at Harper Collins Publishers.

An experienced financial services and operations leader with over 14 years at American Express, specializing in global corporate payments, prepaid solutions, and strategic partnerships across EMEA. Has a strong track record of driving account growth, managing complex multinational relationships, and delivering cross-functional initiatives in highly regulated, global environments.
Since 2016, has served at Adnovate Clinical Development Strategies, where she oversee core operational and financial responsibilities, including payments, operational governance, and financial execution. Is recognized for sound judgment, financial discipline, and ensuring operational efficiency, compliance, and organizational stability.

MA, BA
An organizational and systems minded individual who has experience supporting organizational development of life science consultancies. Has been assisting Adnovate Clinical with operational practices since 2023. Creates processes, systems, and practices that helps Adnovate run efficiently. Motivated, agile, fast learner with a keen eye for process improvement and optimization. MA in Industrial Organizational Psychology.
The Research and Analytics Team
Our Research and Analytics team provides the evidence‑based foundation for critical development decisions. By synthesizing complex scientific and clinical data, therapeutic landscapes, and competitive intelligence, we deliver the deep analytical insights required to de‑risk programs and shape successful clinical strategies.

PhD, MB ChB Medical
Previously a practicing physician, with more than 20years’ experience in drug development. A background in science with a broad range of experience including research in the field of molecular genetics., Experienced in the assessment of potential indications and the production of TPPs and CDPs across a wide range of therapeutic areas, including oncology, nephrology, CV and women’s health. Significant cross-functional clinical research expertise with prior experience in safety management and study oversight. Proven ability to produce high quality, business-valuable deliverables to tight timelines within multifunctional teams. Rigorous scientific approach to problem solving. Experienced author, editor, and reviewer.
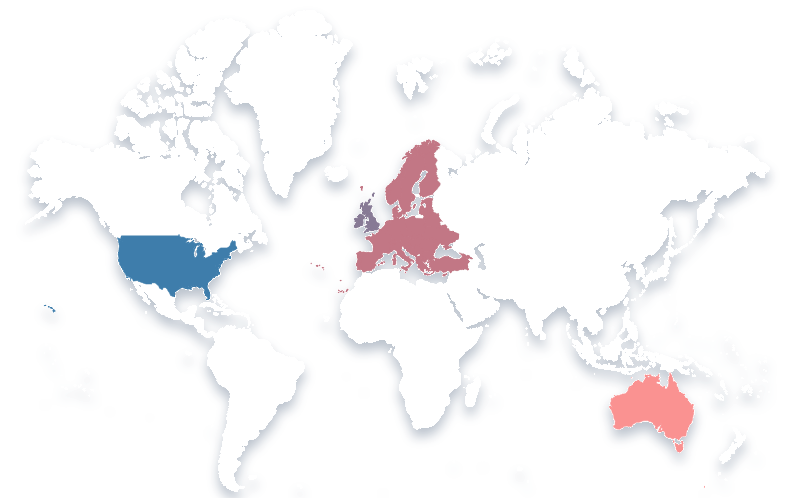
Our Global Reach
Our consultants are experienced drug developers, carefully selected based upon their expertise and reputation. Located across the globe, including North America, Europe, UK, Middle East and Australia, they work by partnering closely with clients to deliver clinical and regulatory strategies that maximize the chances of successfully developing the next generation of medicines.
Clinical Development Strategy
Our cross-functional team of experts work across a range of therapy areas and development phases to define innovative, actionable clinical development strategies that maximize the chances of clinical, regulatory and commercial success.

BPharm, MBBS
Dual qualifications in Pharmacy and Medicine with over 30 year’s experience in clinical development, regulatory approval and commercialization of new therapeutic products. Successfully led four product approvals through the US FDA and EMA including Moraxen, Xyrem and Busulfex. Holds CMO and Board member positions at several life-science companies. Specializes in the early-stage development of therapeutics with an emphasis on novel oncology and hematology products and neuroscience. Worked at renowned companies including Roche, Pfizer, Orphan Medical (US), Genzyme (EU), Arana Therapeutics (AU) and Syneos Health (CRO).

MD
Experienced pharmaceutical industry professional with 30 years of global collaboration in the fields of clinical development, regulatory strategy and submissions, translational medicine, Phase I-IV document preparation, design and study production development, and commercial interaction. Previously Senior Medical Director, Oncology Translational Medicine Unit at AstraZeneca from 2013-2016. Currently holding executive-level positions, including CMO, at several oncology-focused biotechs. Honorary Professor of Translational Medicine, Department of Oncology and Metabolism, University of Sheffield. Also teaching translational medicine and drug development at the University of Manchester.

ND, BASc
Research and development physician/CMO with over 20 years of experience leading global translational, regulatory and clinical teams at start-up biotechs and Pfizer. Early development specialist with expertise in enacting relevant target product profiles and designing innovative and efficient development plans for gene therapies, biologics and small molecules from preclinical through to proof of concept. Accomplishments include design and leadership of pivotal programs, including negotiation of a SPA with FDA leading to market authorization for Veltassa. History of designing development strategy leading to >£50M fundraising rounds. Skilled in portfolio and pipeline strategy and indication prioritization. Broad therapeutic expertise with an emphasis on rare disease (metabolic, CNS, ophthalmology, hemophilia, respiratory indications) and experience in neurological, metabolic and cardiovascular disease and nephrology.

MD
Experienced pharmaceutical physician and executive with over 25 years of experience in clinical development, translational studies, regulatory strategy and submission, commercialization strategies, and fundraising activities. Expertise in a wide range of pharmaceutical products, all stages of development and therapeutic areas with a focus on neurology, psychiatry, analgesia, and orphan indications. Led the clinical development of multiple pharmaceutical products including some to regulatory approvals. Board certified neurologist with academic background in neuro-immunology and neuro-oncology. Held multiple leadership positions in biotechnology, pharmaceutical companies, and contract research organizations, before consulting. Strategic thinker with the ability to tackle complex situations and devise practical and creative strategies for success.

MD, PhD, MBA
Previously a practicing cardiologist and gastroenterologist, with over 20 years of experience in the global pharmaceutical industry, specializing in clinical development (early and late stage), and medical affairs strategy, across a range of therapeutic areas (Rare Genetic diseases, Cardiovascular and Metabolic disorders; Neuroscience; Immunology; Gastrointestinal) for drugs, biologics and medical devices. Global and regional medical director with expertise in life cycle management, FDA and EMA interactions, and successful NDA and BLA submissions. Previous experiences include Global Medical Director and Lead for rare genetic diseases (MPS II, MLD, MPS IIIa) and IBD programs at Takeda/Shire, and Medical Director and Lead for neurosciences/ultrarare diseases at Roche.

MBChB, MRCP
A physician with over 20 years of experience with a proven track record of leadership. Successfully led large global teams within Pharma and Biotech. Currently, providing strategic and operational consultancy expertise to the pharmaceutical, biotech and venture industry. Experienced in developing assets in oncology (small molecule, antibodies, immune modulating and cell therapies) with a focus on immune-oncology across all phases from translational to phase 3, identifying relevant indications and developing biomarker strategies. Past accomplishments include being integral to the successful launch of the Oncology franchise at Johnson and Johnson and serving as their Oncology CREDO champion for 3 years reporting to the Board.

MBChB
UK trained ophthalmologist with industry experience spanning over 20 years. He has held various senior medical management posts including Global Medical Director at Novartis Ophthalmics and Director of Product Development Ophthalmology (EMEA) at Allergan. He has been working as a consultant since 2005 and has worked with several start-up and established companies as well as academic units.

MD, MPH
Trained physician and epidemiologist, practiced in public health and general medicine. More than 25 years of clinical research experience in academia, and the pharmaceutical and biotechnology industry. Strategic advisor on company and product development to executive management teams, company corporate boards, and the investment community. Previously Therapeutic Area Head Ophthalmology and Global Program Medical Director at Novartis Pharma, and Early Development Program Leader Ophthalmology at F. Hoffmann-La Roche. Recently, consulting Chief Medical Officer for early-stage organizations.

MD
Anesthesiologist with 30 years of clinical research experience, 19 of which was in the biotechnology industry including vice president of clinical development at Genzyme. He has diverse therapeutic area experience including Renal, Cardiovascular, Pulmonary, Autoimmune, Rare Genetic Diseases, Biosurgery, Pain and Woman’s Health. Expertise spanning all phases of development (I-IV) and several platforms including proteins, cell and gene therapies, polymers, devices and small molecules

MBChB, MFPM
Experienced senior clinical development physician with a demonstrated history of over 20 years working in the biopharma industry. Skilled in drug development, design and implementation of early to late phase clinical trials, and experience across multiple therapeutic areas, including advanced cell therapies, rare and orphan diseases, oncology, cardiology, neuroscience, allergy, gastroenterology, women’s health, metabolic medicine, diabetes, anti-ageing/longevity, neonatology and autoimmune diseases. Completion of Specialist Training in Pharmaceutical Medicine from Faculty of Pharmaceutical Medicine, Royal College of Physicians UK.

MD, MPH
An experienced clinical research and development leader trained as a physician-scientist with double board certifications (internal medicine and general preventive medicine/public health) and more than 25 years of combined experience in pharmaceutical/biotechnology industry (spanning development programs in small molecules, biologics, and gene/cell therapies ranging from translational medicine to late phase trials and regulatory filings), clinical medicine, and public health (including working on vaccine safety at the CDC). Expertise across a broad range of therapeutic areas including rare diseases (such as lysosomal storage, neuro-degenerative/-muscular), virology/infectious diseases, and immunology (such as rheumatology, dermatology, and GI).

PhD
Senior advisor to multiple biotech companies focused on developing new classes of transformative medicines and delivering technology platforms, with a goal of improving outcomes for patients fighting cancer. Trained as a pharmacologist, toxicologist and embryologist. In 1988, worked in developmental toxicology, first at Zeneca/ICI, and then at Sterling Drug, UK. Held a series of roles of increasing responsibility in preclinical development in the UK, Europe, and the US at Sterling, Sanofi, GD Searle and Pharmacia. Transferred to Pfizer in 2003, led multiple oncology programs and then promoted to franchise head for the heme malignancies portfolio. Moved to Takeda/ Millennium in 2011. Oncology Therapeutic Area Head, Takeda from 2015-2020.

PhD, BPharm, MRPharmS
Extensive experience in drug development consultancy and regulatory affairs with a successful track record building medical and regulatory affairs capabilities for SMEs and startup biotechnology companies across many therapeutic areas, including oncology and neurology. Spearheading regulatory initiatives including preparation and update of key regulatory documents, obtaining CTAs, US IND and fast-track approvals, EU and US Orphan Drug Designations, and leading formal interactions with EMA and FDA. Formerly Head of International Clinical and Regulatory Affairs at Zeneca Pharmaceuticals. Awarded a UK MRC Scholarship and a PhD from the Department of Neurology, Institute of Psychiatry, Psychology and Neuroscience and King’s College Hospital Medical School and the inaugural CPP Post-Doctoral Fellowship at Aston University, UK.

MD, PhD
Drug development expert and formerly a Clinical Assessor at the Austrian Agency for Health and Food Safety and the EMA, with broad expertise across many therapeutic areas, primarily from EMA procedure lead roles (scientific advice procedures, pediatric investigation plans, centralized marketing procedures), with a focus on neuroscience, ophthalmology, pediatric drug development, orphan products, toxicology, metabolic diseases, oncology, dermatology, otolaryngology, rheumatology, immune disorders, parasitology. Previously working in research, teaching and medical practice, then joined the Austrian Medicines & Medical Devices Agency (scientific office) in 2007. Alternate member in CHMP Scientific Advice Working Party 2010-2014, SAWP full member 2014-2023, alternate member of Paediatric Committee (PDCO) from 2008-2013 and a PDCO full member 2013-2023.

PhD
Statistician with over 25 years of experience, primarily in the pharmaceutical industry. Expertise in the design and analysis of clinical trials, providing guidance to sponsors on their development strategies and on appropriate study designs and analyses across a broad range of therapeutic areas. Experienced in interacting with the FDA and European regulatory agencies, providing statistical input to regulatory briefing documents, meetings and responses. Keen interest and experience in the application of the recent ICH E9(R1) addendum on estimands and sensitivity analyses. Member of the PSI (Statisticians in the Pharmaceutical Industry) Scientific Committee since 2014, and the EFSPI/EFPIA Estimand implementation Working Group since 2020. Excellent training and presentation skills with an ability to clearly communicate complex statistical concepts to non-statisticians.

PhD
Experienced pharmaceutical industry professional with over 30 years of experience in clinical development. Innovative, outcomes-oriented, clinical development leader, inspiring and driving clinical development strategies and operations to successfully progress investigational products through development, meeting key business requirements. Strong track record of successful team and systems establishment and global project delivery. Previous roles include VP/Head of Clinical Development in biotech and global pharma. Broad therapeutic area experience including the development of novel agents for the treatment of CNS, CVRM, GI, Oncology, Ophthalmology, Respiratory, and Immune-Inflammatory conditions.

ScD
Clinical development professional with over 20 years’ experience including 11 years at Pfizer as a biostatistician and team lead for late-stage endocrine products. Expertise in traditional and adaptive clinical trial design, regulatory interactions, and due diligence evaluations. Experience across all phases of development from early oncology and rare disease studies to post-marketing commitments, acting as independent reviewer, DSMB/DMC voting member, sponsor’s representative at FDA AdComm meetings, providing responses to FDA/EMA/PMDA, reviewing in-licensing and co-development business opportunities. Lecturer of Research Design in the Applied Analytics graduate program at Columbia University.

BA
Clinical research professional with >30 years of medical writing experience in the industry, CRO, and independent consulting setting across a broad range of therapeutic areas, notably oncology. Managed medical writing groups in large and mid-sized CROs. Lead writer on multiple successful investigational new drug applications and marketing applications. Extensive experience authoring phase I-IV clinical study protocols, subject information and consent forms, clinical study reports, nonclinical and clinical CTD modules, integrated summaries of safety and efficacy, investigator brochures, regulatory meeting briefing documents, and patient narratives as well as SOPs and other operational documents. Interactively works with colleagues and collaborators to efficiently produce high-quality documents. Commitment to the highest scientific and ethical standards.

PhD, MB ChB
Previously a practicing physician working in the health care sector for over 20 years. A scientist with a broad range of experience including basic research in the field of molecular genetics, assessing potential oncology indications for early clinical development, and significant cross-functional clinical research expertise in safety management and study oversight across a wide range of therapeutic areas including oncology, nephrology and women’s health. Proven ability to produce high quality, business-valuable deliverables to tight timelines within multifunctional teams. Rigorous scientific approach to problem solving. Experienced author, editor, and reviewer.

PhD
A qualified professional certifying Investigational Medicinal Products for Clinical Trials in both the UK and EU. Over 30 years industry experience across a broad range of therapeutic areas including oncology and both Autologous and Allogeneic Advanced Therapies including CAR-T, Viral Vector gene delivery and CRISPR. Involved in many novel drug development successes including the first gene therapy to be approved in the European Union. Previously a PhD student in the Hematology department at the Royal Free Hospital and winner of the 1988 Margaret Kenwright award of the British Blood Transfusion Society for research.

PhD
Over 36 years’ international experience in development, implementation and execution of global regulatory, quality, clinical development strategy, reimbursement/ pricing negotiations and post-launch clinical evidence programs for medical devices, IVDs, drug/biologic-device combination products and biologics. Liaison and negotiation with key regulatory authorities and supporting expert bodies (US FDA OCP, CBER and CDRH divisions, European Commission and national drug / device agencies, Notified Bodies, Chinese NMPA, Japanese PMDA/MHLW). Worked with both large and small medical device and pharma companies in regulatory, quality and clinical roles including. Hands-on experience in a variety of therapeutic areas and across different technologies and types of medical products including software-controlled devices and standalone software (including mobile apps).

BSc
Clinical Development professional with more than 30 years’ experience, a sustained record of success, and a strong understanding of R&D trends, strategy and markets. Resourceful, with a broad range of scientific and business expertise. Experiences include founding member and VP Clinical of a small biotech. Well versed in facilitating in-depth research, improving processes, analyzing data and delivering results as well as overseeing complex projects, utilizing change, technical, risk and resource management expertise. Strong leadership skills, adept at building and managing key relationships including stakeholders and clients, effectively translating requirements and overseeing all issues through to completion.

PhD
Clinical pharmacology leader with 25+ years of global experience driving PK/PD strategy, study design, and regulatory submissions from first-in-human through Phase 3. Proven track record in leading cross-functional teams across clinical pharmacology, biostatistics, clinical operations, and regulatory affairs to deliver optimized dose selection, accelerate development timelines, and ensure compliance with FDA, EMA, and Health Canada requirements. Expert in clinical pharmacology GAP analysis and interaction with regulatory bodies. Skilled in selecting and managing Phase 1 CROs, overseeing non-compartmental and population PK/PD modeling, and integrating pharmacometric analyses into development programs. Experienced in clinical pharmacology NDA/BLA modules review in oncology, neurology, and other diseases, with a history of successful IND, NDA, and IB authorship and defense.

MSc
Biostatistician with over 15 years of experience providing methodological advice to the pharmaceutical industry and academia from preclinical to Phase III across a broad range of therapeutic areas. Extensive knowledge on complex problems and methods especially for innovative designs, multiplicity issues, interim analyses, and oncology designs. Leadership across multiple activities including the choice of study design, sample size calculations, simulations, randomization and CRF validation, statistical sections of the protocol, statistical analysis plan, interpretation, visualization and valorization of results including publications. Experienced DMC member and trainer in biostatistics and advanced methodologies.

MsC
Global Pharmaceutical Statistician with over 25 years’ experience of the design and analysis of Phase1-IV clinical trials, including contributions to clinical development plans. Highlights include: experience of several FDA EU and ROW submissions, leading the statistical function on regulatory rapid response teams and extensive FDA interactions from End-of-Phase 2 to post-approval commitments.

MD MBA
An experienced medical executive with a strong background in oncology, rare diseases, and drug-device combinations. A solid track record of leading R&D teams globally, guiding treatments from early development to clinical trials, including therapies for glioblastoma and CAR-T innovations. Known for strengths in strategic planning, budget management, and team collaboration, has contributed to successful regulatory approvals across various therapeutic areas. With a medical degree and an MBA from Warwick Business School, blends medical knowledge with business strategy.

MD, PhD
Board certified in Internal Medicine & Endocrinology, with >30 years’ experience using innovation in trial design to improve POS and reduce costs, leveraging expert advice from KOLs, across multiple TAs including metabolism/endocrinology (19 years), oncology (13 years), CV (4 years), CNS and dermatology (3 years each), and ophthalmology (2 years). Successfully led 2 drug approvals (Nesina/ diabetes) and Azedra (pheochromocytoma), and contributing to others e.g. Contrave (obesity), Pylorify (PSMA imaging agent, prostate cancer). Experience extends from translational science and early-phase human trials to pivotal NDA-enabling studies employing efficient trial designs e.g. CRM, BOIN for phase 1, adaptive designs for phase 2/3, real world outcomes and “large simple studies”. Also supported Medical Affairs, Health Outcomes and market access functions through KOL panels, publications and customized clinical studies to support benefit claims.

MD, PhD
Experienced pharmaceutical industry professional and CMO with over 20 years of clinical development experience from translational to successful launching of small molecules and biologicals. Experience includes overseeing strategic clinical development and early and late-phase clinical trial design, and medical monitoring, spanning phases 1-4 across the US and Europe for a broad range of Therapeutic Areas: autoimmune diseases, orphan diseases, immunology, chronic inflammatory diseases, transplantation, infectious diseases. Previously a practicing physician and an academician working in infectious diseases & immunology. Then a drug developer in autoimmune and orphan diseases at Protein Therapeutics, Guildford Pharmaceuticals, UCB, Genzyme and Argenx.

MA
Senior writer and editor with expertise across diverse disciplines. More than 25 years of experience in medical writing for industry, CROs, and NGOs across a broad range of therapeutic areas, including manuscripts, phase I-IV clinical study protocols, subject information and consent forms, clinical study reports, IMP Dossiers, CTD modules 2.5, 2.7.3, and 2.7.4, Integrated Summaries of Safety and Efficacy, Investigator’s Brochures, briefing documents, and patient narratives. Proven ability to collaborate with cross-functional teams in global settings, producing high-quality documents that meet client expectations. Commitment to the highest scientific and ethical standards. Reputation for clear communication and keen appreciation of precision, nuance, and clarity.

MB, MSc, FFPHM, FFPM
Senior Pharmaceutical Physician with extensive Clinical Development, Medical Affairs and Senior Management experience. Specialist (equal to Board Certification) in Public Health Medicine (Epidemiology, Health Economics, Critical Appraisal, Data Analysis) and Pharmaceutical Medicine (Regulatory, Pharmacology, Stats, Clinical Development, Marketplace, Safety). Development experience from translational to phase III in multiple therapeutic areas; neuroscience, oncology, inflammatory disease, immunology and neuropathic pain; rare and orphan diseases; small molecules and biologics as well as clinical strategy, regulatory, business development, drug safety, health economics and reimbursement. Good understanding of both biotech and bigger company cultures. Educational supervisor, Faculty examiner and appraiser.

PhD
Consulting statistician using modern trial designs to develop next generation evidence-based medicines. Over 20 years’ experience, primarily supporting pharma and medtech companies with regulatory interactions for early & late phase adaptive and Bayesian designs, machine learning for biomarker discovery, diagnostics using signals and images, and training & developing statistical software. Biostatistician at the NY Academy of Medicine from 1999 working in HIV research alongside clinicians, public health workers and patient advocacy groups. Biostatistician at Nestle Research Center from 2010 developing medical devices and designing non-clinical experiments and innovative clinical trials for medical food, microbiome & genetic studies. Various positions of responsibility in advanced statistics & data science at prominent CROs from 2013-23.

MB, BS, MRCP
A UK dually accredited Clinical Pharmacologist and Pharmaceutical Physician experienced in small molecule, biopharmaceutical and gene therapy development, working directly with small start up companies as well as with medium and large sized pharmaceutical companies. Ex VP and development leader at GSK as well as ex-head of the GSK European Cardiovascular Clinical Team in the Cardiovascular and Metabolic Medicines Development Centre with European clinical development responsibilities for all licensed products in Phase 2/3 development. Expertise includes rare diseases, metabolic, cardiovascular, renal, urology, ophthalmology, immuno-inflammation, haematology and oncology therapeutic areas.

MD, MBA, MS(Nutr)
Biotech and pharma executive with >25 years’ experience in clinical research and development of small and large molecules, cell and gene therapies. Proven leader of translational, early and registration clinical development programs in primary and specialty care TAs, including metabolic, endocrine, hematologic, pulmonary, CV, rare genetic metabolic and nephrological diseases. Clinical leader for multiple regulatory submissions (IND, NDA, CTA, PIP, MAA) and interactions. Major accomplishments include: application of functional neuroimaging to phase 1 and 2 studies for investigational therapies for obesity and diabetes; NDA and regulatory negotiations for the first CV protection label for a therapy for diabetes (empagliflozin); novel clinical development strategy and phase 1/2 study design for an investigational gene therapy for nephropathic cystinosis (acquired by Novartis). Author of more than 100 peer reviewed papers and book chapters.

MD, PhD
Cardiologist with over 20 years of drug development experience including early and late phase clinical development strategies at the program and portfolio level, trial design, feasibility planning, regulatory interactions and medical monitoring, assuming responsibility for ethical and medical aspects of studies. Provides therapeutic training, benefit/risk evaluations and drug safety expertise for drugs, diagnostics, and devices in development and during market authorization. Extensive experience interacting with health authorities, IRBs/ethics committees, investigators, key opinion leaders and data monitoring committees.

MSc, MDRA
Dedicated drug development professional with 25+ years in the biopharma industry and regulatory agency (EMA). In-depth experience in global regulatory affairs. Strong understanding of current principles of all areas of drug development, life-cycle management & market access for medicinal products, including drug-device combinations. Development of regulatory strategies for all types of medicinal products, regulatory project management, dossier compilation (eCTD, IMPD), and submissions. Broad therapeutic area experience including oncology, rheumatology, autoimmune and rare diseases (orphan drugs). Planning, preparation and handling of scientific advice/Pre-IND procedures and agency meetings, regulatory submissions in support of clinical trials (CTA, IND), pediatric development plans (PIP, iPSP), specialized procedures (e.g. Orphan Drug, Fast Track), initial marketing authorizations (MAA, NDA/BLA) as well as post-authorization procedures.

PhD, MSc
Senior medical writer with more than 15 years of experience, providing a broad range of clinical and regulatory documents across a range of therapeutic areas including documents in CTD format, regulatory response documents, briefing packages, scientific advice documents, clinical protocols, clinical study reports (Phase 1-4), investigator brochures, patient information leaflets, CTD abstracts, formulary packs, study narratives, manuscripts, abstracts and posters for International scientific meetings. Particular expertise in Phase 1/clinical pharmacology studies in both healthy volunteers and patients. Maintains familiarity with current industry practices, regulatory requirements, and guidelines related to medical writing. Proven track record for the interpretation and presentation of scientific data into clear well organized documents and presentations. Strives to provide quality documents on time.

PhD
A clinical pharmacologist with over 30 years of experience in the Pharmaceutical Industry, and a PhD in pharmacokinetics and drug metabolism. Former clinical pharmacologist at Pfizer working across numerous therapeutic areas, where roles included Clinical Pharmacology Lead for early and advanced candidates through to regulatory approval in major markets. Co-founder of Valley Writing Solutions Ltd in 2007, who work with Pharmaceutical and Biotech companies, and specialise in clinical pharmacology focused medical writing and consulting. Document specialities include CTD Modules 2.7.1 and 2.7.2, clinical protocols, clinical study reports, Investigator Brochures, briefing books, regulatory responses, and manuscripts. Highly experienced in working collaboratively across multi-functional teams to meet client goals.

PhD
Physician scientist with over 15 years of global medical practice spanning Brazil, the UK, Canada, and the USA. Internationally recognized for impactful contributions to critical care, immunology, and machine learning, evidenced by numerous peer-reviewed publications. Highly skilled in building and leading interdisciplinary research teams, leveraging advanced machine-learning techniques to extract insights from high-dimensional molecular and clinical data to translate research into personalized therapies to meet each patient’s unique clinical needs. In recent years, leveraged extensive experience in a dual role for precision immunology therapeutics, actively contributing to both clinical development and research endeavors.

MD
Previously a practicing physician trained in internal medicine and a specialist in nephrology, with >20 years of drug development and medical affairs experience in Pharma and Biotech organizations. Prior leadership roles at Genzyme, Alexion and several start-up companies. Experience spans across a variety of therapeutic areas including Nephrology, Hematology, and multiple rare diseases from pre-clinical to launch.

MD, PhD
A driven passionate senior executive – pharmaceutical physician-scientist with demonstrated track record of successful blockbuster product registrations and launches in USA, EU, Australia and other markets with over 25 years of experience. Previously VP, Head of Neurology and IBD Ozanimod, BMS, VP, Head of Neurology US and Global Medical Affairs, Celegene, and Global Brand Medical Director, Neuroscience, Novartis. Extensive Academic Research experience in immunology with focus on autoimmune diseases. A co-author on more than 150 scientific publications including more than 60 pubmed listed manuscripts. Designed and wrote research proposals to national and international granting bodies and attracted a significant amount of funding for research projects in immunology.

PhD
Biotech executive with over 27 years of experience in drug development, leading cross-functional, global projects from drug candidate selection in research, through PoC to Phase III, approval and commercialization, including three successfully launched products. Background in pharmacy and pharmacology research to compliment both strategic and operational leadership of multifunctional teams. An accomplished presenter and a respected executive leader, enabling effective management and leadership at both the program and board level. Recent accomplishments demonstrate an ability to work effectively within small companies and teams, driving agility and being externally focused, adapting to the needs of the patient and the business environment.

MD
Experienced pharmaceutical industry professional with over 20 years of experience. Previously CMO of Eloxx pharmaceutical, responsible for IND submission and conducting trials in multiple rare diseases, and Senior Global Project Head in Rare Disease Clinical Development at Sanofi-Genzyme, overseeing key functions responsible for advancing a number of investigational therapies along with full approval. Held several positions of increasing responsibility with expertise across a range of therapeutic areas including cardiovascular disease, nephrology and immunology. Also held clinical development roles at Ionis and Takeda, where he worked on approval of a CKD anemia compound.

DPhil (Oxon), MPharm (Hons)
CMC and regulatory strategy consultant working in the industry for close to 20 years, including 9 years in research and development focusing on advanced biological therapies. Strong expertise in the technical scientific, development and regulatory aspects of in vivo and ex vivo gene therapies, particularly for cancer and degenerative disorders. Specific experiences include CMC (quality) data-driven gap analyses and global regulatory and development strategies for in vivo and ex vivo genome editing, virally vectored gene therapy products, oncolytic viruses, cell-based immunotherapy, genetically modified cells for tissue regeneration, stem cell-based products, and genetically engineered immune cells targeting cancer cells.

MSc, Biostatistician
A seasoned Consultant Biostatistician with over 20 years of expertise in the pharmaceutical industry and clinical research. Delivering comprehensive biostatistical support across all stages of clinical trials, from study design to analysis and reporting. Known for leading biometrics activities and overseeing CRO deliverables, provides clients with high-quality, strategic insight into clinical development, with experience spanning across a wide range of therapeutic areas and most phases of drug development. Specific interest and expertise in process optimization, project management, enhancing data interpretation and cross-functional collaboration.

PhD, MRPharmS
Regulatory professional with >18 years diverse experience in devising and implementing innovative regulatory strategies aligned with the needs of the business, market, and patients. Seasoned knowledge of the EU/EEA, UK, US and ICH regulations across all clinical development phases (1-4). A key strength is my ability to proactively create and lead strategies to influence regulators, fostering robust working relationships with authority contacts. Proven track record of successfully navigating and managing complex and novel regulatory issues. Adapt at leading, planning and managing the development and timely submission of high quality documents to regulatory authorities (e.g., scientific advice, IMPDs, ODDs, PIPs/iPSPs, INDs/CTAs and MAAs/NDAs).Diverse experience includes working with novel drug therapies both small molecules and biologicals, particularly in rare diseases and oncology complemented by recent, valuable experience in the rapidly evolving field of AI-driven drug development.

MBA
Biopharmaceutical strategist with more than 20 years of experience across global, regional, and local affiliate roles in the biotechnology industry. Trained in Genetics and Business, with a career spanning basic research at UdelaR and clinical research at Brigham and Women’s Hospital before transitioning into industry. Held positions of increasing responsibility at Genzyme, Biogen, and Vertex, including early commercialization strategy and development planning from discovery through Phase IV. Served as regional LATAM integration lead during a major post‑acquisition transition at a global biotech, directing lifecycle planning, regulatory filing and launch sequence strategy across specialty therapeutic areas, including neurology, cardiometabolic and biosurgery. More recently, led market entry in South America for two rare disease U.S‑ and EU‑based companies as General Manager, including new affiliate buildup, go‑to‑market planning and execution, and direct P&L management. Brings a strong track record of integrating scientific and commercial perspectives to guide biopharma innovators through international market expansion, with integrity and patient centricity.
Translational Strategy
Our translational experts leverage their extensive scientific and development expertise to ensure delivery of pre-clinical and early development plans that maximize the probability of regulatory success and provide early indications of activity to increase value creation.

PhD, FRC Path
35 years of experience in pharmaceutical drug Discovery and Development and former Vice President of Safety Assessment in the UK for AstraZeneca. Experience consulting and supporting small, medium and major pharmaceutical companies, Universities and Charity organizations devoted to the Discovery and Development of new medicines. Experienced in Drug Discovery and Development within a wide range of therapeutic areas including, respiratory, inflammation, neuroscience and cardiovascular and oncology. Responsible at AstraZeneca for the non-clinical safety development of the full oncology portfolio including registration of Irressa and Olaparib. Experienced in the development of small molecules, peptides, monoclonal antibodies and gene therapy projects in oncology.

DVM, PhD
More than 30 years of experience in the pharmaceutical industry in discovery and development. Certified specialist in Pharmacology and Toxicology as well as Translational Medicine. In-depth scientific knowledge in Oncology (Head of Takeda Safety Pharm and in vivo oncology), Gastroenterology, Inflammation, Respiration, Metabolic Diseases (Type 2 Diabetes, Obesity, NASH), Pain, CNS, Safety Pharmacology and Toxicology, and Orphan Diseases. Extensive project management experience, bringing more than 15 compounds up to Phase 2 and working with international teams with multicultural backgrounds. 50+ publications and 27 patents.

BSc
Expertise in preclinical drug metabolism and pharmacokinetics as applied to drug discovery and development gained through 24 years’ experience with Pfizer Global R&D in various management roles and senior scientific roles in the department of Pharmacokinetics, Dynamics & Metabolism, working on programs and internal projects such as sildenafil for erectile dysfunction and pulmonary hypertension, dofetilide for cardiac arrhythmia, maraviroc for HIV, licensing evaluations with external companies, and the harmonization of global scientific best practices. Contributing author to global regulatory submission dossiers and query responses in areas of scientific expertise for sildenafil, dofetilide, maraviroc and voriconazole. Industry and academic tutor in fields of drug metabolism, pharmacokinetics, toxicokinetics and physicochemistry as applied to drug discovery. Author of over 50 scientific papers.

PhD
A research scientist with pharmacology and biochemistry background and over 45 years of experience in the pharmaceutical industry from bench scientist to scientific management. Currently providing translational and pharmacology consulting for biotech and pharma companies in early drug discovery and clinical development. Previous roles include Research manager within GSK research with project leadership and management responsibilities for numerous projects and senior scientist role in a biotech company. Research experience spans exploratory and target discovery projects and clinical development across a wide range of therapeutic areas and indications including allergic inflammatory, respiratory, gastrointestinal, cardiovascular, oncology and orphan diseases.

PhD
An applied mathematician/statistician with extensive experience working for a variety of healthcare industries from academia (NHS) to biotechs and large pharmaceutical companies (AstraZeneca Innovation Award 2012, UK 3R’s award 2011), supporting investment (biotechs) and drug development decisions within the preclinical and clinical space (QSP/preclinical PKPD/population PKPD/biostatistics) with a focus on Oncology. Developed numerous technical skills including numerical analysis differential equations, (hierarchical) mixed-effects modelling, statistical learning techniques, longitudinal and time-to-event analysis techniques. Academic experience includes supporting an Oncology NHS trust to improve their data analysis techniques of observational data in order to generate new biomarker/end-point hypotheses and improve clinical decision making. Over 75 peer-reviewed publications, primarily in statistical modelling for Oncology.

PhD
Over 28 years of experience working in the pharma and biotech industry with expertise identifying and translating candidates from Discovery Research through Clinical Development. Providing real scientific and strategic solutions to address the challenges of drug discovery and development as well as pragmatic data driven program leadership identifying and progressing candidates through discovery and development/regulatory milestones. Proven scientific and strategic leadership in gene therapy, cell therapy, oligonucleotides, small molecule and biologics. R&D experience in numerous therapeutic areas inclusive of specialty and rare diseases, genetic diseases, CNS, neuromuscular, immunology, ophthalmology, metabolic, oncology and cardiovascular.
Clinical Operations Strategy
Our team have significant experience across all phases of drug development, assisting sponsors with the selection and oversight of CROs internationally, ensuring high-quality trials are delivered on time, on budget, and in compliance with industry regulations and guidelines.

PhD
A committed clinical operations professional with proven leadership ability and a progressive, inclusive management style. Over 25 years of clinical research experience including VP, Head of Development Operations for a mid-tier pharma organization and Director of Project Management for a CRO. Gained experience across all study phases and a range of TAs including GI, pain (neuropathic and nociceptive), oncology, renal and diabetes. An excellent communicator with a demonstrated record of innovative problem solving, extensive project management, and leadership experience within the pharmaceutical and medical device industries.

MSc, MBA
Experienced Life Sciences Expert and PMP®-certified Project Manager specializing in Life Sciences Advisory and Business Process Management. Background as a Paramedic and Molecular Biologist, with over a decade of experience working in the pharmaceutical industry, including roles in CROs, Pharma, and Consulting. Clinical experience includes Phase I-IV clinical trial monitoring, primarily in CNS and oncology. Post-marketing expertise involves establishing pharmacovigilance and quality assurance frameworks, data migration, and digitalization. Key skills include outsourcing strategy, vendor and alliance management, IT solution implementation, and Change and Project Management.

PhD
An established pharmacovigilance professional with nearly 20 years of experience in pharmacovigilance both in medicines regulation as head of the risk management and pharmacoepidemiology group at the MHRA, and in the pharmaceutical industry as vice-president and global head of Risk Management Centre of Excellence and Pharmacoepidemiology at Takeda. Her experience as a regulator and as a senior executive in industry provides her with a unique ability to guide pharmacoepidemiology studies, benefit risk assessments and pharmacovigilance processes to successful conclusions.

MSc
Experienced clinical trial professional with over 17 years’ experience as a global clinical project manager, specializing in patient recruitment. Worked with CRO, pharma, academic and commercial stakeholders in cross-functional roles. Experienced in a wide range of therapeutic areas, phase I-IV trials and all stages of trial life cycle from writing successful funding applications to study close-out. Key skills: patient recruitment, study set-up and delivery to time and budget, trial materials, medical writing, clinical site engagement, strategic and creative thinking, troubleshooting, vendors and PPI (patient, public involvement) activities. Excellent verbal (e.g. leading kick-off meetings, patient focus groups, investigator/client meetings and bid defense) and written communication (e.g. recruitment plans, protocols, PIS, ICF, SOPs, CSR and materials aimed at patients and clinicians).

MA
Accomplished quality management professional with over 20 years’ experience in clinical and PV quality management, quality systems, computer systems, operations, and project management, GCP (GCLP)/GVP/ CSV, Quality Management Systems, Standard Operating Procedures, Training, Audit Management & Conduct, Inspection Readiness & Hosting, Risk Management, Change Management, Quality Control, Document Control, Project Planning, Consulting. Critical thinker, committed to identifying compliant, practical, pragmatic and fit-for-purpose solutions to quality and operational challenges. Possessing a sharp eye for detail, but focused on the big picture. Excellent written and verbal communication skills. Knowledgeable, creative and flexible.

BPharm
A Clinical Project Director with extensive experience in clinical research and development spanning over 25 years within the Biotech, Pharmaceutical and Clinical CROs. Previous positions include Clinical Project Director at Quintiles (UK) and Clinical Project Manager at Innovex, Icon and GSK (legacy Wellcome Foundation). Currently provides clinical project management support to pharmaceutical companies, working across a spectrum of small and large organizations, including Biotech start-ups and SMEs. Specializes in the set-up, management and quality provisions for the conduct of clinical studies from early phases of drug development through to pivotal studies and post-marketing trials across a broad range of indications including orphan diseases, inflammatory conditions and chronic immune mediated disorders.

BSc, PgCert
An established Pharmacovigilance Scientist educated and trained in the UK with over 15 years of direct pharmacovigilance experience in the pharmaceutical industry across a broad range of therapeutic areas, including oncology, opioid-dependent pain, and neurology. Experienced across the breadth of product development, from early phase human studies to the post-marketing setting. Adept at conducting and reviewing benefit/risk assessments as part of both routine signal management and aggregate reports.

BSc
Program Director with over 25 years’ experience in the clinical research industry (CRO and Pharmaceutical/Biotech Companies) in phases I-IV study set-up to final report and regulatory submission. Has spent the past 5 years as a consultant to start-up companies in early phase development for rare disease. Experience: Phase I healthy volunteer and complex early phase patient studies, Neurology, Infectious Disease, Rare Disease, Cardiovascular.

MSc
A science graduate with more than 20 years of clinical research operations experience within Pharma and small Biotech organizations, in roles up to Clinical Operations Director. Experience includes 10 years of line management and more than 15 years of clinical project/program management. Therapeutic areas cover extensive experience in oncology, including immuno-oncology, as well as gene therapy/ATMPs, GI and ophthalmology, and all phases from First In Human to peri-approval work.

BSc
An efficient, dedicated and enthusiastic clinical operations leader with a keen eye for process improvement. Over 15 years’ experience within clinical research, overseeing and executing multiple global clinical development programs including vendor selection and oversight, process optimization and resource utilization. Wide therapeutic experience with a specialist interest in rare disease and oncology. Experienced across all areas of clinical studies phases I-IV. Excellent leader and communicator with a demonstrated record of innovative problem solving and extensive project management experience within the pharmaceutical industry.

PhD, BSc
A PhD pharmacist with more than 25 years experience in clinical operations in the CRO environment serving both Pharma and Biotech organisations with recent Executive Director roles in Clinical Operations, Study Start Up and Site Contracts. An accomplished leader with expertise in change management, strategic planning and process improvement, driving growth and operational excellence. Proven track record in cross-functional global leadership, delivering results through effective relationship and team building. Excellent organizational and communication skills with an independent, proactive mindset and the ability to solve problems in complex and fast-moving environments.
Our Team at Work
We can provide individual functional experts or assemble bespoke selected teams with experience and expertise directly relevant to the needs of our clients. We tailor our work and leverage our significant drug development experience to produce high quality outputs through the application of strong science, innovative clinical trial design and a deep understanding of regulatory and commercial landscapes to generate maximum value for our clients’ assets.
Excellence
Accountability
Integrity
Innovation
Creativity
Equality
Respect
Teamwork