Small capital-restrained early-stage biopharma company partners with Adnovate Clinical
Biopharma Introduction
Japan-based biotech, a spin-out from Takeda, focusing on rare diseases looking for support to translate their lead asset to the clinic.
The Adnovate team functioned as an extension of the GEXVal Inc/GEXVal AU Pty Ltd internal team supporting not only the clinical study execution but strategic planning of the product and additions to the quality management system as needed. GEXVal and Adnovate foster an excellent team with partners in Australia, sharing the same goal to deliver innovative medicines to patients and families who live with rare diseases.
Juran Kato | CEO GEXVal Inc.
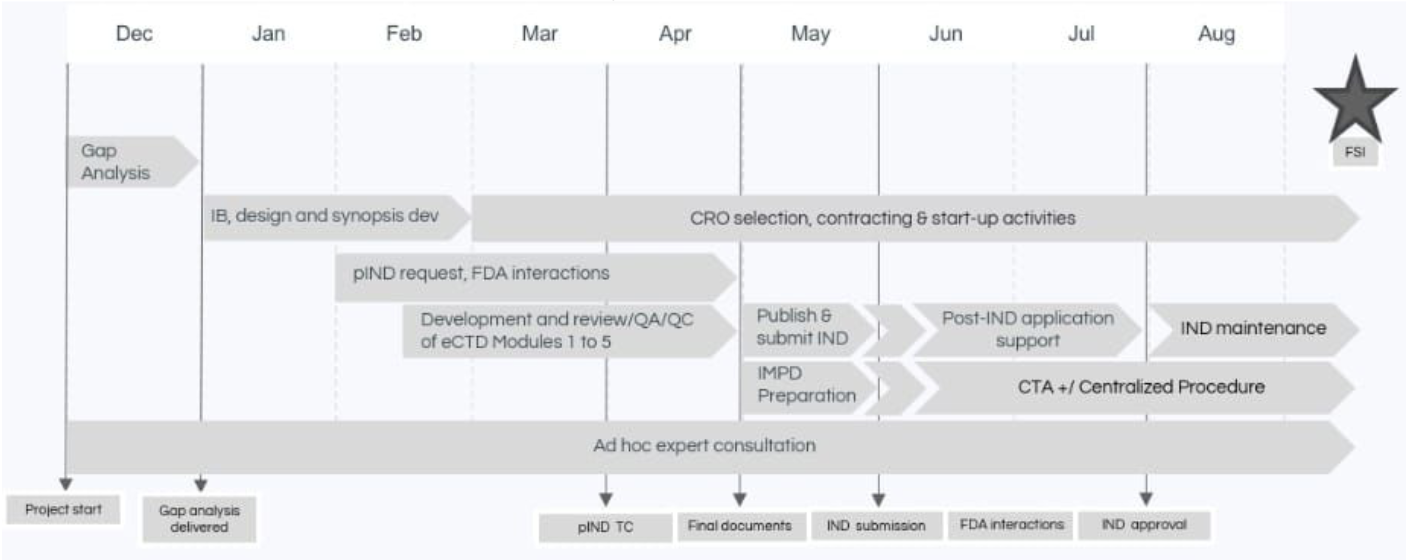
Adnovate Clinical’s Solution
Functional leadership through provision of interim CMO and clinical operations and statistical staff to support clinical development activities. Adnovate Clinical designed and guided the development of the translational, clinical and operational plans for a phase 1 study, successfully transitioning it into clinic, producing a high-quality protocol with a complex biomarker approach incorporating innovative trial and statistical methodology and providing the guidance for selection and then oversight of the CRO to produce key study documents and processes, study setup and execution using a risk-based management approach. The study ran to time and the team helped manage the CRO budget tightly and represented the sponsors interests to the highest level at alltimes.